The ‘Why’?
FLFE’s goals were threefold:
- To create and validate a survey instrument for collecting representative FLFE customer experience data.
- To identify most frequent FLFE customer experiences associated with the effectiveness of the FLFE environment.
- To investigate whether FLFE customer experiences occur due to a placebo effect.
The ‘Who’?
338 existing FLFE customers with varying durations on the FLFE service.
The ‘How’?
The Customer Experience Survey (CES) instrument was announced on the FLFE Facebook group and via email campaign, encouraging FLFE customers to participate.
The ‘When’?
Data collection occurred in September of 2021.
The ‘Where’?
Data collection occurred online using Microsoft Forms.
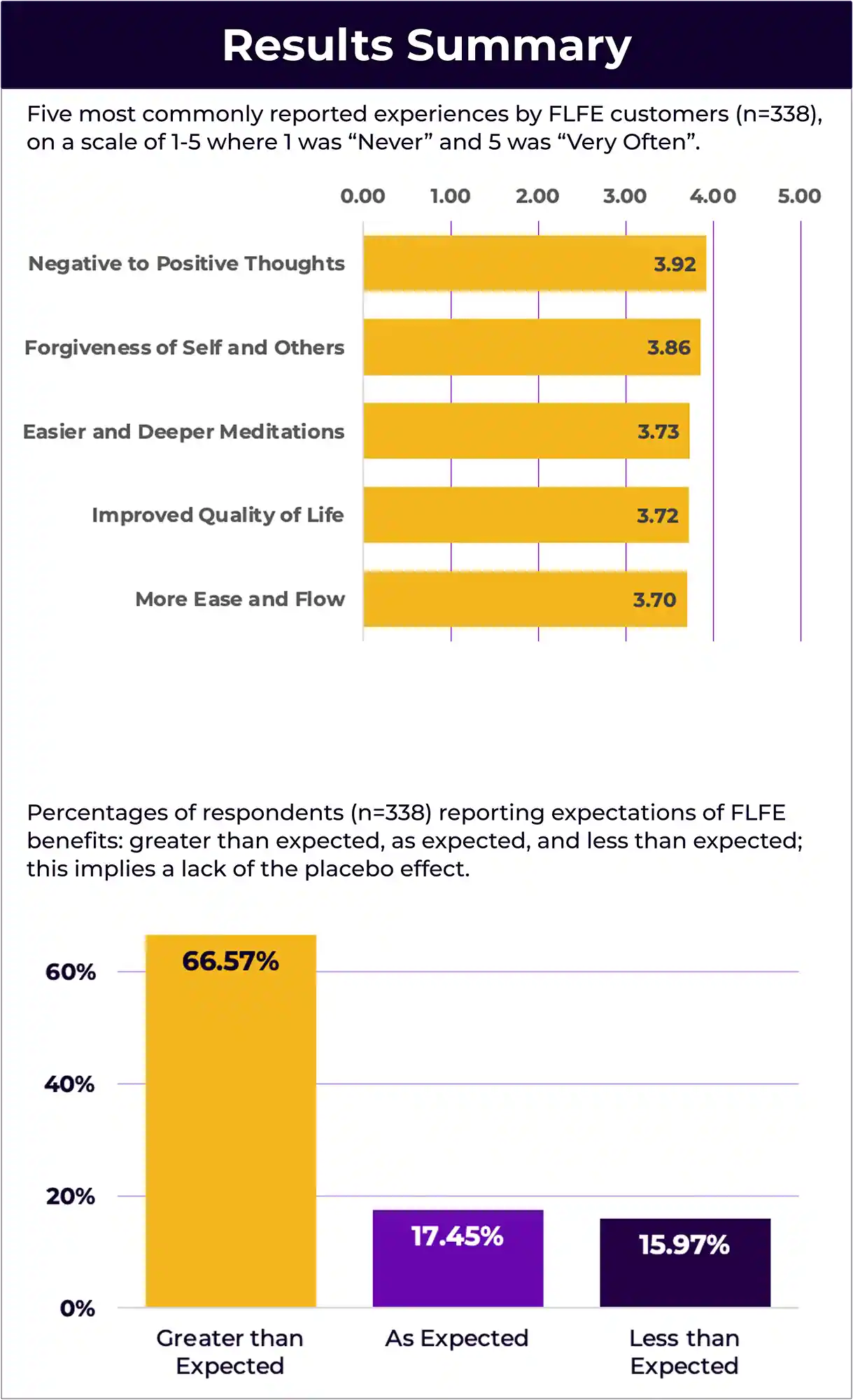
Abstract: Focused Life-Force Energy (FLFE) customers often spontaneously share the beneficial effects they experience from being in the FLFE environment. We analyzed four years of customer experience data to find the experiences that were most common. A survey instrument was developed to evaluate the experiences, their replicability, and the relationship between expectations and actual experiences. This was a large-scale Phase 1 exploratory large-scale research project with a representative sample of 338 FLFE customers. The majority (66.57%) of the respondents reported that their experiences of the benefits were greater than they expected implying a lack of the placebo effect. Overall, this study shows the effectiveness of FLFE for the majority of its users.